Key Takeaways:
- Innovative Cancer Treatment: A groundbreaking project, PDC*neo+, is set to revolutionize colorectal cancer treatment with a personalized therapeutic vaccine.
- Significant Funding and Collaboration: The project, involving key biotechnology and technology companies and academic centers, receives a substantial €8.1M funding from the Walloon region and BioWin.
- Potential and Impact: PDC*neo+ promises not just improved patient care but also significant economic and societal benefits, including job creation.
Introduction to a New Era in Cancer Treatment
In an era where medical advancements shape the future of healthcare, a significant breakthrough in cancer treatment emerges. The development of PDCneo+, a personalized therapeutic vaccine for colorectal cancer, marks a milestone in the field of immunotherapy. Spearheaded by PDCline Pharma, this project represents a collaboration of biotechnology experts, technology innovators, and academic researchers, united in the fight against one of the most prevalent cancers worldwide.
The PDC*neo+ Project: An Overview
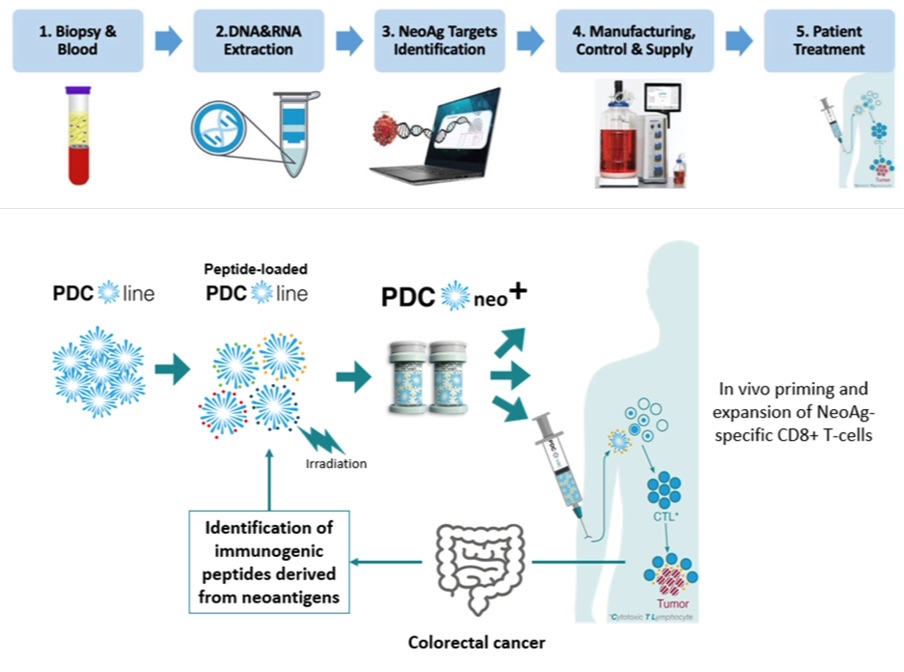
PDC*neo+ is not just another cancer treatment; it’s a beacon of hope for colorectal cancer patients. By leveraging the unique properties of neoantigens, this vaccine aims to target cancer cells more effectively than ever before. It’s an adjuvant treatment designed to prevent cancer relapse in high-risk patients, addressing the gap in post-surgery and chemotherapy care.
Unprecedented Funding and Collaboration
The Walloon region and BioWin’s investment of €8.1M in this project underscores its potential impact. With a total budget of €12.5M, the project brings together the innovative minds at PDC*line Pharma, OncoDNA, salamanderU, and academic institutions like UCLouvain-IREC/MIRO and ULB-BCTL. This consortium is a testament to the power of collaborative effort in advancing medical science.
The Science Behind PDC*neo+
The core of PDCneo+’s innovation lies in its use of neoantigens, which are uniquely suited to each patient’s cancer profile. This personalized approach ensures that the vaccine targets the cancer cells more precisely, enhancing the effectiveness of the treatment. The project’s goal is to establish the clinical feasibility and safety of PDCneo+ in a phase I trial, setting the stage for a new standard in cancer care.
Economic and Societal Impact
Beyond its medical significance, PDC*neo+ is expected to have a substantial economic and societal impact. The project will create and maintain around 34 full-time jobs over its three-year duration. This not only represents a boost to the economy but also a step forward in establishing a robust healthcare ecosystem centered around innovative cancer treatments.
The Voices of Innovation
Eric Halioua, CEO of PDCline Pharma, expressed his enthusiasm, noting, “The subsidies and collaborative effort of the partners in the consortium will speed-up the launch of a first phase I clinical trial with PDCneo+, positioning our technology in the very promising field of neoantigen-based cancer vaccines.”
Jean-Pol Detiffe, founder of OncoDNA, highlighted their role in the project: “It acknowledges our recent proficiency in the discovery of neoantigens and our robust experience in liquid biopsy monitoring.”
Claude Dedry, CEO of salamanderU, emphasized their contribution: “We are bringing the production of PDC*Neo+ closer to the patient, strengthening our position as a designer and manufacturer of innovative isolators.”
Academic partners from UCLouvain and ULB also shared their excitement about contributing their expertise to this groundbreaking project.
Conclusion: A New Dawn in Cancer Treatment
As PDCneo+ moves from concept to clinical trial, it stands as a beacon of hope and innovation in the fight against colorectal cancer. This collaborative effort not only represents a significant scientific advancement but also a model for future healthcare initiatives. With its potential to change lives, PDCneo+ is more than just a medical breakthrough; it’s a step towards a future where cancer treatment is more effective, personalized, and accessible.
Additional Resources and Contacts
For more information about PDC*line Pharma, OncoDNA, salamanderU, and the academic institutions involved, visit their respective websites. Media and analysts seeking further details can contact Andrew Lloyd & Associates.
Source: https://www.pdc-line-pharma.com/
Sign up to our newsletter & get the most important monthly insights from around the world.
Ready to Amplify Your Brand with Business Today?
Discover the power of sponsored articles and partnerships to reach decision-makers, professionals, and a dynamic audience. Learn more about our advertising opportunities and connect with us today!
Click here to explore our Promotion & Sponsored Articles page.
Are you looking to make an impact? Contact us at [email protected] to get started!