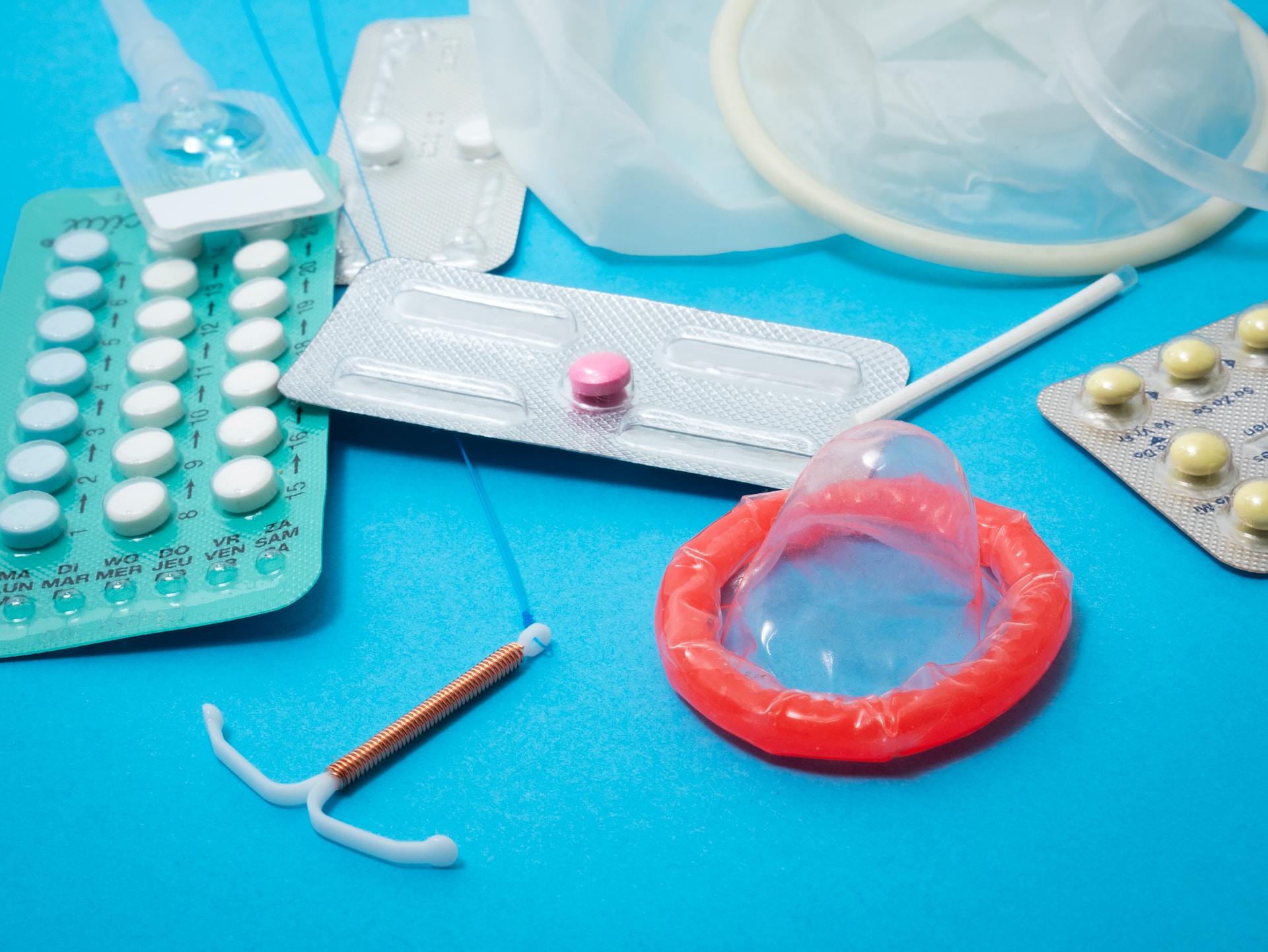
Advanced BioScience Laboratories (ABL), a leading global Contract Development and Manufacturing Organization (CDMO) for biotherapies and viral vector vaccines, has been awarded a seven-year contract by the Division of AIDS (DAIDS) within the National Institute of Allergy and Infectious Disease (NIAID) at the National Institutes of Health (NIH). The contract, named ‘Resources to Advance Pediatrics and HIV Prevention Science (RAPPS)’, aims to provide critical support for preclinical research, development of pediatric formulations, and advancement of next-generation non-vaccine Biomedical HIV Prevention products (nBPs). This significant contract carries a total potential funding value of $98.9 million.
Key Takeaways:
- ABL secures a seven-year, $98.9 million contract from DAIDS at NIH.
- The contract aims to support preclinical research, pediatric formulation development, and advancement of next-generation HIV prevention products.
- ABL’s expertise will contribute to reducing HIV infections and addressing the needs of underserved populations, including pediatric patients.
Advancing HIV Prevention and Pediatric Treatment
ABL’s role under the RAPPS contract is multifaceted. The organization will provide critical services to DAIDS, including preclinical gap-filling, animal models, bioanalytical services, product manufacturing, and scientific and quality/regulatory support. The focus will be on supporting preclinical research, developing pediatric formulations, and advancing next-generation non-vaccine Biomedical HIV Prevention products. These products, such as IntraVaginal Rings (IVRs), long-acting injectables, and Multipurpose Prevention Technologies (MPTs), have the potential to target not only HIV prevention but also pregnancy and Sexually Transmitted Infections (STIs).
ABL’s long-standing collaboration with DAIDS reflects their commitment to HIV research and development. Since the 1980s, ABL scientists have played a crucial role in advancing HIV pathogenesis research, developing therapeutics, and contributing to the development of vaccines.
Addressing Global HIV Challenges
The World Health Organization (WHO) estimates that there are over 38 million individuals living with HIV worldwide, with approximately 4,000 new infections reported daily. Developing safe, effective, and accessible products for HIV treatment and prevention is critical in reducing the global burden of the disease. Unfortunately, women and girls continue to be disproportionately affected, accounting for 53% of all people living with HIV. Access barriers and poor product adherence further exacerbate the challenges faced by at-risk populations.
The development and approval of HIV treatment and prevention products specifically tailored for the pediatric population remain a pressing need. Currently, only a fraction of the AntiRetroViral (ARV) drugs approved for adults are approved for children under the age of two. ABL’s expertise and support will enable DAIDS to advance the development and availability of innovative HIV prevention products for the underserved pediatric population.
About the National Institutes of Health (NIH)
The NIH is the primary federal agency responsible for conducting and supporting medical research in the United States. Comprised of 27 institutes and centers, the NIH investigates the causes, treatments, and cures for a wide range of diseases. Its programs encompass basic, clinical, and translational research, with the goal of improving patient care and public health.
About ABL
ABL, Inc. is a renowned global contract research and manufacturing organization focused on advancing therapeutics, vaccines, and biological products. With extensive experience working with industry, government, and academic entities, ABL offers a comprehensive range of services. Their global Good Manufacturing Practice (GMP) facilities meet stringent US and European regulatory standards, enabling the development and production of virus-based oncolytic therapies, gene therapies, and viral vaccines. ABL’s services include bulk drug substance manufacturing, live virus fill/finish, process and assay development, as well as preclinical and clinical sample processing and testing through their immunological laboratories. ABL is part of the Institut Mérieux, a group of companies dedicated to developing translational science for better patient care globally.
Sign up to our newsletter & get the most important monthly insights from around the world.
Ready to Amplify Your Brand with Business Today?
Discover the power of sponsored articles and partnerships to reach decision-makers, professionals, and a dynamic audience. Learn more about our advertising opportunities and connect with us today!
Click here to explore our Promotion & Sponsored Articles page.
Are you looking to make an impact? Contact us at [email protected] to get started!